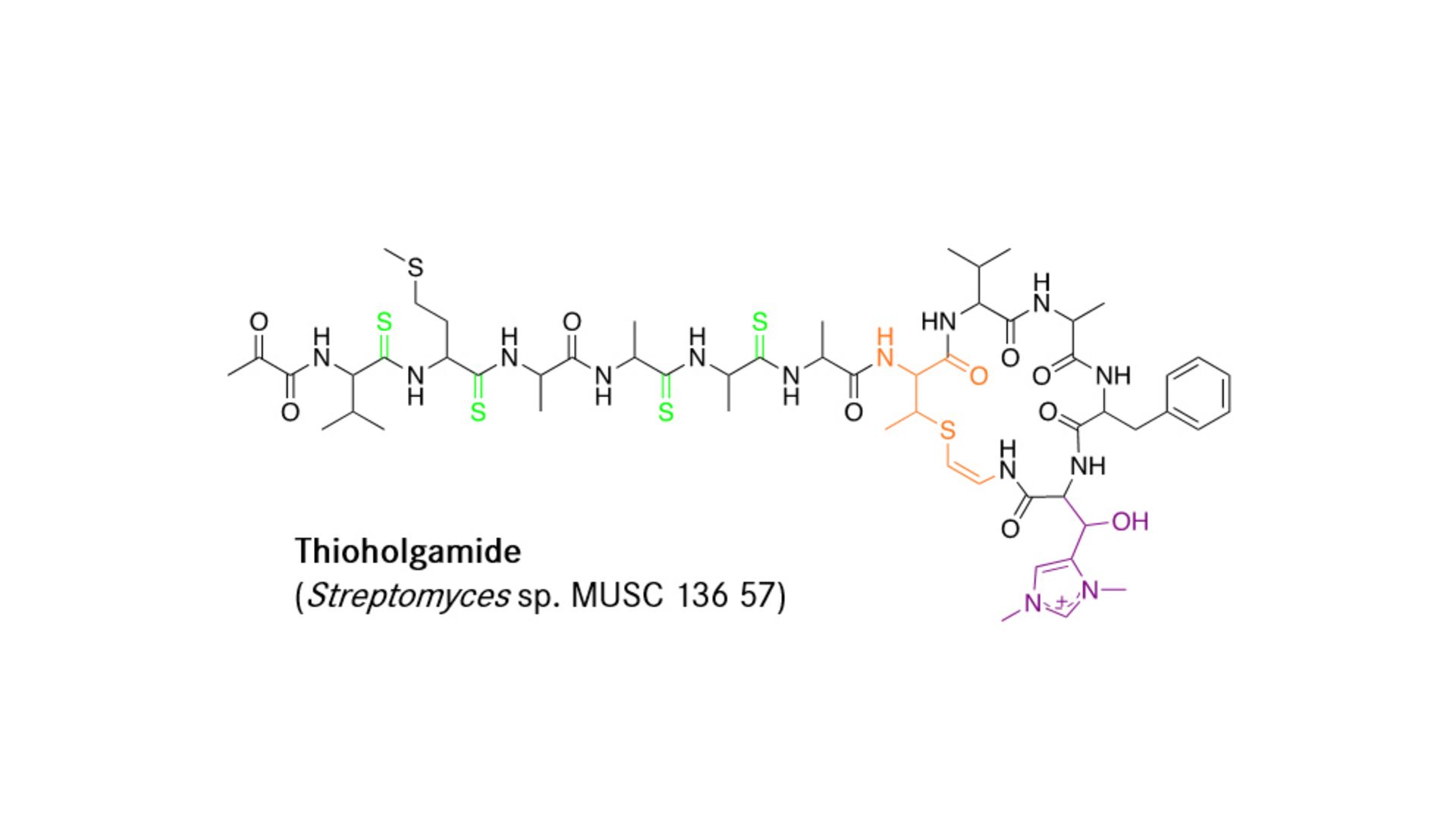
Saarbrücken, 22 March 2022 – Scientists from the Department Microbial Natural Products have elucidated the biosynthetic pathway of Thioholgamides. These peptides have potent activity against several cancerous cell lines. The novelty of their work lies in the in vitro reconstitution of the biosynthetic pathway, which allows for the development of novel derivatives with optimized properties. Their work was published in the Journal of the American Chemical Society. In the interview Asfandyar Sikandar tells us more about his work.
In your paper you elucidated the bioynthesis of thioholgamides, which belong to the so called “RiPP” family. What are RiPPs and what is the biological function of the thioholgamides?
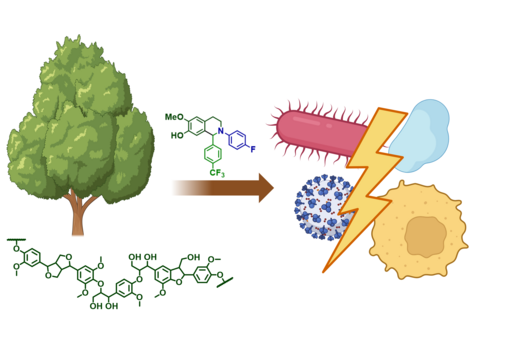
Ribosomally synthesized and post-translationally modified peptides (RiPPs) are a large group of natural products with remarkable structural and functional diversity. The biosynthetic system of RiPPs typically involves a short precursor peptide - consisting of a leader and core motif - and nearby processing enzymes that recognize the leader and perform chemical modifications on the core to give the mature scaffold. Thioholgamides are a subgroup of RiPPs that have been shown to possess potent antiproliferative and pro-apoptotic activity in several cancer cell lines. Despite the promising anticancer activity, we lacked a comprehensive understanding of their biosynthesis, making it a very exciting research project for us to pursue.
How did you reconstruct the biosynthetic pathway? What benefits can you derive from a detailed understanding of this process?
Since their identification back in late 2000s, work at HIPS and labs around the globe have been putting the pieces of this “biosynthesis puzzle” together. However, the major bottleneck to reconstruct the entire pathway in a tube was the availability of active and correctly folded pathway-specific enzymes in our hands. Once we figured out how to overcome this hurdle, it was relatively straightforward to reconstitute the entire biosynthetic pathway. This work has allowed us to resolve ambiguities and correct inaccuracies that exist in previous in vivo studies. In short, we now have a much better understanding of the how thioholgamides are made in nature. With this knowledge, we are in a much better position to create designer compounds with improved activity against cancer cells. Since similar enzymes are also utilized by other RiPP pathways, we hope that our work will help elucidate the biosynthesis of other natural products as well.
In your article, you mention that histidine bis-N-methylation takes place. Could you explain this process in more detail and why is it so special?
This modification is installed by a methyltransferase encoded in the biosynthetic gene cluster of the thioholgamides. Interestingly, this modification has not been reported previously for any other natural product and it also introduces a permanent positive charge on the compound. Electrostatic interactions can be vital for the interaction of the natural product with its target protein, but it remains to be seen if this is also the case for thioholgamides. We are currently trying to shed more light on the potential importance of methylation on thioholgamide bioactivity.
Have you already been able to generate new compounds with improved properties?
HIPS in conjunction with Prof. Andriy Luzhetskyy from Saarland University are actively working on this topic. I think we will see the first batch of optimized thioholgamides soon.
Dr Alwin Hartman and Dr Yannic Nonnenmacher conducted the interview.
Original publication
Sikandar, A., Lopatniuk, M., Luzhetskyy, A., Müller, R., Koehnke, J., Total In Vitro Biosynthesis of the Thioamitide Thioholgamide and Investigation of the Pathway. Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c00402.