Saarbrücken, 17 April 2020 - Scientists at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) have developed a strategy to administer antibacterial agents in a way that makes them many times more effective. Their results give rise to hope in the fight against hospital infections and antibiotic resistance. The study has been published in the international edition of the journal Angewandte Chemie/Applied Chemistry. The HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in Braunschweig in cooperation with Saarland University.
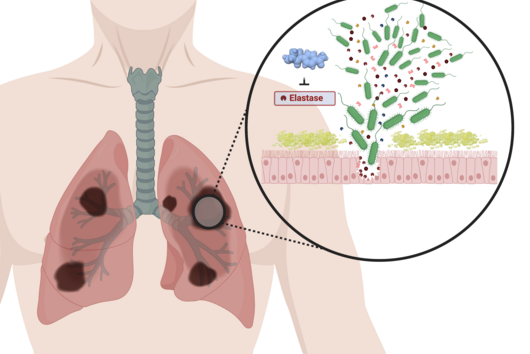
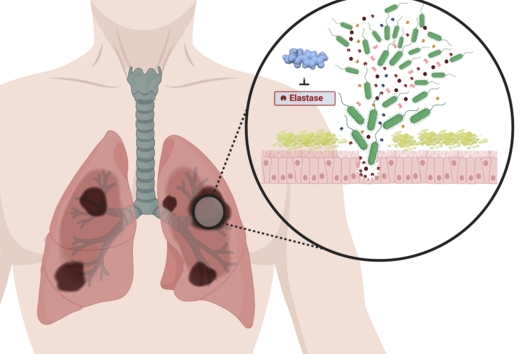
The world is currently fascinated by pharmaceutical research, which is supposed to free us from the burden of the new coronavirus SARS-CoV-2. In addition to the search for a vaccine, the further development of other drugs is of crucial importance, especially now and well beyond the crisis. Many of the most severely ill COVID-19 patients have to be treated with antibiotics, for example, because a chronic pre-existing disease or a superinfection with bacteria puts a strain on the lungs and drastically worsens the course of the disease. The treatment of these patients is made more difficult by the resistance of the germs to antibiotics, which is constantly emerging. A typical colonizer of the lung is Pseudomonas aeruginosa. The bacterium is known to cause hospital infections and make life difficult for people with cystic fibrosis. It forms a protective layer of complex, long-chain molecules in affected tissues. This biofilm forms a barrier against antibiotics, much to the suffering of the patients.
Professor Claus-Michael Lehr has been researching biological barriers for decades. "The intestinal wall, the skin, the air-blood barrier in the lungs or even biofilms: They all stand in the way of the active substance," says the head of the department "Drug Delivery" at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS). "In order to reach the site of action, drugs need a suitable vehicle designed for the specific active substance and the respective barrier".
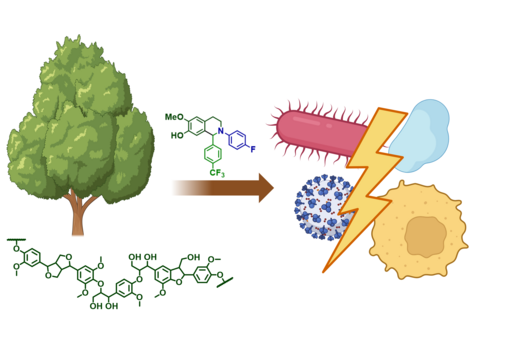
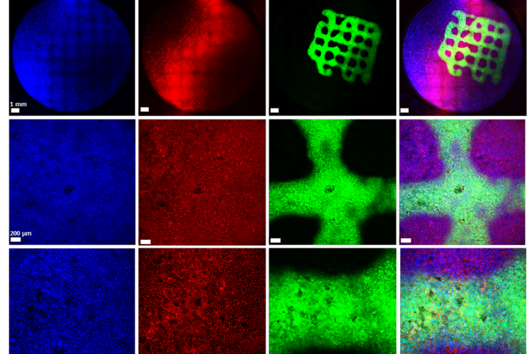
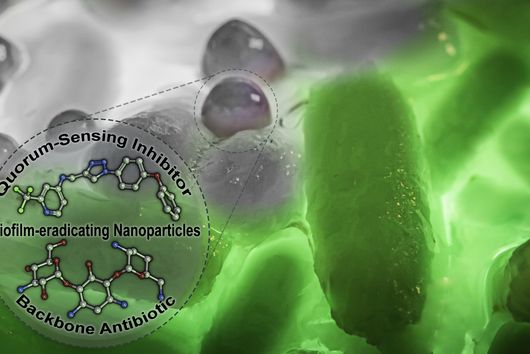
In cooperation with French pharmacist Patrick Couvreur in Paris, PhD student Duy-Khiet Ho produced special nanoparticles that were supposed to transport antibiotics into the biofilm formed by pseudomonads. He synthesized a novel amphiphilic carrier molecule, which self-assembles to nanostructures capable of being loaded with both water and fat-soluble drug molecules.
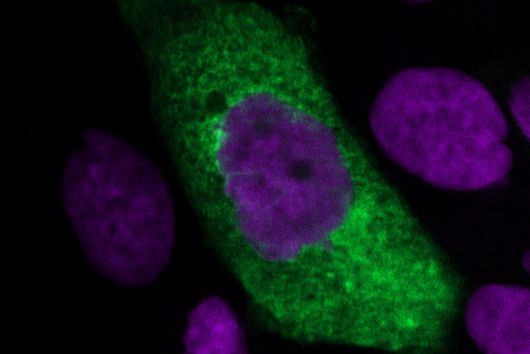
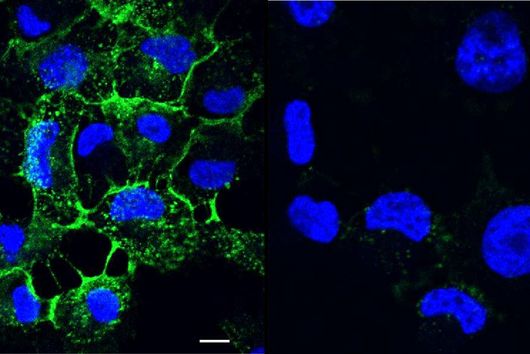
Under the microscope, the scientists were able to observe how the particles infiltrate and penetrate a cultivated bacterial biofilm. However, when they put the antibiotic Tobramycin, usually used to fight Pseudomonas aeruginosa infections, into the nanoparticles, they did not see any increased effect against the germs in the biofilm. Dr Brigitta Loretz, who developed the method together with Lehr, explains the reason for this: "In the biofilm, the bacteria shut down their metabolism. The antibiotic, which actually blocks protein production, can therefore not have a major effect. If you throw a stick into a gearbox that is standing still anyway, you will not achieve a braking effect.” That is why the researchers took advantage of another point of attack of the germs: quorum sensing. This describes the mechanism by which bacteria determine whether they have reached a certain population density. Only then does it make sense for them to form a biofilm. Quorum sensing regulates, among other things, the production of the polymers required to build the biofilm. Prof Rolf Hartmann and Dr Martin Empting in the neighbouring department "Drug Design" at HIPS are investigating novel substances that are able to flip an important switch in this mechanism. The Lehr group used one of these substances: They loaded their nanotransporters with the quorum sensing inhibitor QSI(1) in addition to the antibiotic Tobramycin. If they treated biofilm cultures with the double-loaded particles, a fraction of the antibiotic concentration was sufficient to kill the bacteria and dissolve the biofilm. "We turn off quorum sensing directly in the biofilm," says Loretz. "The bacteria kick up their metabolism and the antibiotic takes effect."
From a medical point of view, it is of immense importance to be able to treat infections in a targeted manner with the help of low concentrations of antibiotics. "In this way, we prevent bacteria from developing resistance to the active substances," explains Loretz.
"We have produced a first generation of anti-infective nanocarriers," said Lehr who is now further developing the novel nanoparticles. In their cultivated biofilm cultures, Lehr and his colleagues are not only able to observe the particles. They can also measure exactly how many of the active substances have penetrated the biofilm. "This is a clear advantage over tests in laboratory animals," he says. His strategy is to investigate as many parameters of a new active substance as possible "in the test tube". To this end, the passionate inventor develops complex models, preferably from human cells, which reflect the conditions in the body as closely as possible. "It's like a modular system," he says. "In vitro, for example, I can cultivate different cells in different combinations to understand the role of each cell type." Only recently, Lehr and his colleagues published the first protocol for a three-dimensional cell culture model of respiratory tract infections in which he cultivates mucosal and defence cells together with biofilm-forming bacteria. In such a model, different inflammation parameters can be observed and antibiotics tested simultaneously. He believes that after extensive in vitro studies, animal experiments are only necessary for confirmation. His goal is to test new active substances in humans as quickly as possible. "After all, we do not want to cure mice," he says.
Patrick Couvreur and Claus-Michael Lehr are involved in the EU-funded International Training Network (ITN) ”NABBA”: Design and Development of advanced NAnomedicines to overcome Biological BArriers and to treat severe diseases. The development of the inhibitor QSI(1) was funded by the German Center for Infection Research (DZIF).
ORIGINALPUBLIKATION:
Duy-Khiet Ho, Xabier Murgia, Chiara De Rossi, Rebekka Christmann, Antonio G. Hüfner de Mello Martins, Marcus Koch, Anastasia Andreas, Jennifer Herrmann, Rolf Müller, Martin Empting, Rolf W. Hartmann, Didier Desmaele, Brigitta Loretz, Patrick Couvreru, Claus-Michael Lehr: Squalenyl Hydrogen Sulfate Nanoparticles for Simultaneous Delivery of Tobramycin and Alkylquinolone Quorum Sensing Inhibitor to Eradicate P. aeruginosa Biofilm Infections. Angew. Chem. Int. Ed. 2020; doi: 10.1002/anie.202001407