Saarbrücken, 18 May 2022 - In old age, memory performance declines in most people, often to such an extent that it has a massive impact on their everyday lives. Bioinformaticians at Saarland University and Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), in a joint study with Stanford University, have now identified a protein with a rejuvenating effect that influences the process of declining brain performance in old age. The HIPS is a site of the Helmholtz Centre for Infection Research (HZI) in collaboration with Saarland University. Their research results were published online in the scientific journal Nature.
There are many causes of age-related diseases - and they often lead to considerable limitations in the everyday lives of those affected. One of the most common symptoms is a gradual decline in cognitive capacity, which is often later paired with senile dementia. Researchers at Saarland University are therefore interested in the question of which new substances, in addition to a healthy lifestyle, can help us stay fit in the brain for as long as possible. They are also looking for active substances that can cure symptoms in patients who are already affected.
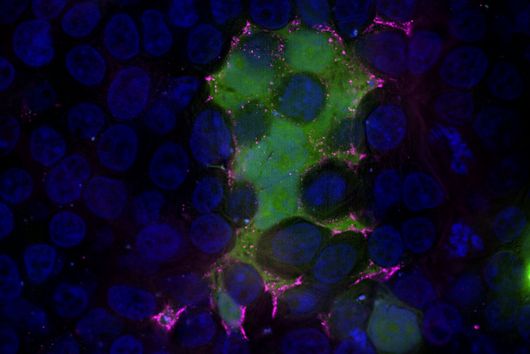
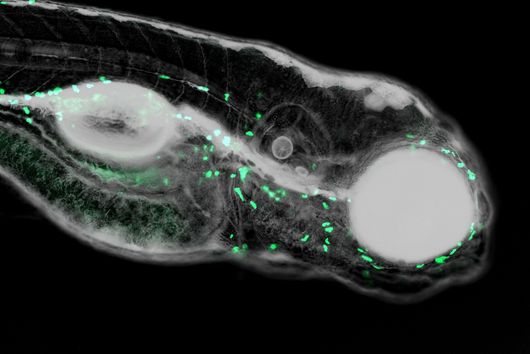
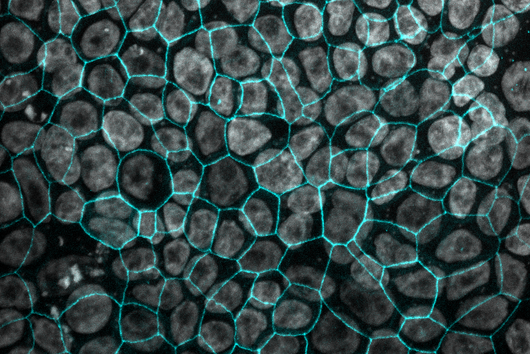
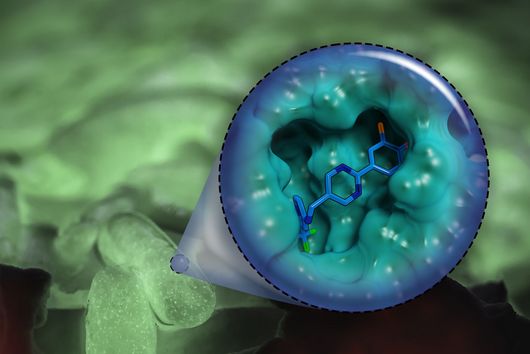
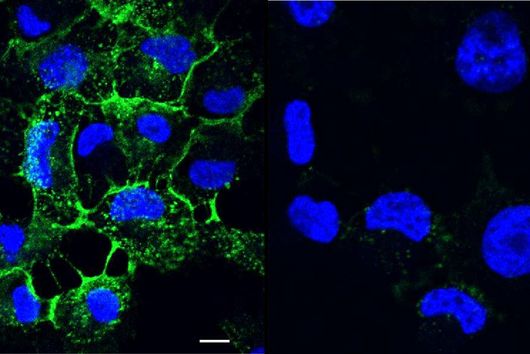
In a study together with researchers from Prof Tony Wyss-Coray's group from the Department of Neurology at Stanford University in the USA, which has now been published in the renowned journal Nature, bioinformaticians Andreas Keller and Fabian Kern describe the discovery of such a physiologically effective substance. Andreas Keller is a professor of clinical bioinformatics at Saarland University. His former PhD student Fabian Kern has recently started his independent research group at the Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), where he will investigate infections on a single cell level with spatiotemporal resolution to drive the development of novel antiinfectives. Together, they have contributed significantly to deciphering the actual mechanism behind the mode of action of the so-called signal protein Fgf17. This protein comes from a family of cellular growth and development factors. The protein, which is soluble in the cerebral fluid (also known as cerebrospinal fluid - CSF), specifically docks onto corresponding receptors of the brain cells, in particular the so-called oligodendrocytes from the glial cell family. These are indispensable for the electrical signal conduction of our neurons, but can lose in number and function with increasing age. One of the best-known autoimmune diseases directly attributable to a loss of oligodendrocyte function is multiple sclerosis. CSF contains a complex mixture of multiple nutrients that supply the cells in the central nervous system, as well as various proteins with still unknown functions.
The naturally occurring protein described in the study leads to a partial reversal of the observed ageing processes through a reactivation of oligodendrocytes and, in addition, to a significant improvement in cognitive weaknesses. This could be demonstrated by transferring spinal fluid from young laboratory mice to their older conspecifics by transfusion. The Saarbrücken bioinformaticians have described the transcription factors and genes involved in this specific molecular process in more detail. However, there are still many more questions to be answered before a possible therapy in humans can be developed. The first step was to identify suitable drug candidates, which usually proves to be extremely difficult and time-consuming. In this case, the bioinformaticians were able to make such a discovery possible with their methodological knowledge, such as in high-throughput sequencing. "Interdisciplinary research of this kind with selected proximity and potential for clinical use are among the university's goals in the NanoBioMed area. In this way, pioneering innovations are carried from the campus to the whole world," says Professor Andreas Keller with regard to the study now published in the journal Nature. The print edition of the article finally appears in the issue of 19 May.
ORIGINALPUBLICATION
Iram, T., Kern, F., Kaur, A. et al. Young CSF restores oligodendrogenesis and memory in aged mice via Fgf17. Nature (2022). DOI: 10.1038/s41586-022-04722-0