Technology Platforms
Adequate equipment provides the basis for accomplishing cutting-edge research. The HIPS comprises several state-of-the-art technology platforms so our scientists can focus on their research without having to deal with technical limitations.
NMR Spectroscopy
NMR spectroscopy is one of the most powerful tools for the identification and verification of molecular structures. We use our NMR spectrometers for routine measurements (e. g. structure verification in organic synthesis, standard 1D and 2D experiments) as well as for de novo elucidation of unknown structures and advanced, non-standard techniques. Our NMR department includes three different NMR devices (Bruker Biospin, Ettlingen), each of them having its own application focus:
Avance III Spectrometer, UltraShield 500 MHz, He-cooled 5 mm TCI CryoProbe (‘inverse’)
Avance III Spectrometer, Ascend 700 MHz, He-cooled 5 mm TCI CryoProbe (‘inverse’)
Avance Neo Spectrometer, UltraShield Plus 500 MHz, N2-cooled 5 mm Prodigy BBO-CryoProbe (‘observe’) with autosampler.
Zebrafish Facility
Zebrafish (Danio rerio) are one of the most important non-mammalian vertebrate model organisms. Especially the larvae are of interest as the embryonic development is very rapid, and the larvae are relatively large, robust, and transparent.
Since 2017, the Zebrafish Facility at HIPS is fully functional. It has a system volume of 3,500 liters and a total capacity of > 25,000 animals. Two wild type strains as well as several mutant or transgenic models are currently available. In our BSL2 laboratory, two microinjection stations comprising of fluorescent stereomicroscopes with pressure injectors and micromanipulators are available.
At HIPS, we mainly use the zebrafish larvae for studying natural products for their potential toxicity, maximum tolerated concentration (MTC), and importantly, we developed a number of infection models for early in vivo screening. As part of drug metabolism and pharmacokinetic (DMPK) studies, we recently established a method for studying the spatial distribution of compounds and their metabolites in zebrafish larvae using Mass Spectrometry Imaging (MSI).
Protein Crystallography
Obtaining atomic resolution structures of proteins through x-ray crystallography is a multi-step process and we cover all of these steps at the HIPS. First, the protein of interest needs to expressed in a soluble, folded form. We have bacterial and mammalian expression systems at our disposal that allow for rapid and easy testing of a large variety of expression conditions. Once suitable conditions have been identified, we can scale the procedure to purify anything from µg to >100 mg. Robotics allow us to cover many crystallization conditions and obtain initial crystals using very little sample (150 nL per condition). These are then optimized, also with the help of robotics to yield high-quality crystals suitable for structure determination. Data collection at synchrotrons enables us to determine the atomic-resolution structure of the protein of interest, if desired in complex with other proteins or small molecules.
Biophysical Methods
At HIPS, new anti-infective agents are developed in an iterative process comprising multiple optimization steps. In this context it is indispensable to investigate biomolecular interactions such as the binding of a drug (ligand) to its target structure, usually a defined protein. A precise qualitative as well as quantitative statement about the biomolecular interaction of two molecules is a prerequisite for the further optimisation process.
At the biophysical testing platform at HIPS, various types of technologies are used to investigate biomolecular interactions, e.g. for the quantification of binding affinities. These comprise Surface Plasmon Resonance (SPR), Differential Scanning Fluorimetry (DSF), MicroScale Thermophoresis (MST) and Isothermal Titration Calorimetry (ITC). The choice of the biophysical methods to be used generally depends on the molecular properties and availability of the interaction partners.
Imaging
The Imaging platform available at HIPS offers cutting-edge technology for both functional and molecular imaging. Combining fluorescence (normal and confocal) and scanning electron microscopes, this platform allows visualising and characterising micro and nanoscale drug delivery systems, as wells as physiological, biochemical and infective phenomena in in vivo/in vitro systems (e.g. living or fixed cells, tissues, biofilms, etc.).
The fluorescence microscope (Nikon Eclipse Ti- S) is an optical microscope that uses fluorescence to observe both biological and inorganic samples. The confocal microscope (Leica DMi8 inverted microscope) also uses fluorescence optics to resolve detailed structures but in several optical planes, bringing the opportunity to obtain high-resolution 3D reconstructions of the samples. Labelling the samples with fluorescent dyes, the fluorescence microscopes allow us to visualise e.g. cellular compartments, cellular growth patterns, biofilm structures or the biodistribution, internalization and subcellular location of drugs and carriers inside the cells or within the tissue.
The scanning electron microscope (SEM EVO HD15) produces high-resolution images and gives information of the sample topography (e.g. shape and size of particles) and composition (e.g. distribution of the nanoparticle size).
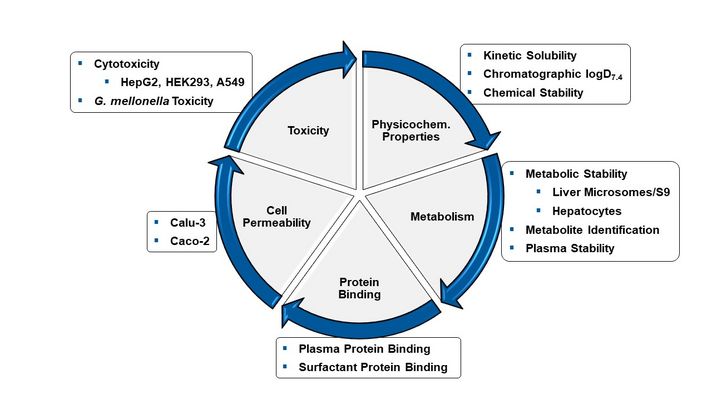
ADMET-Facility
In the translational phase of transferring academic drug-discovery projects into treatment options (from bench-to-bedside), we put a strong focus on the determination of in vitro ADMET properties (absorption, distribution, metabolism, excretion and toxicity). For successful drug-discovery, it is of vital importance to consider these pharmacokinetic properties of a potential new drug candidate early on in a discovery campaign. Towards this end, HIPS has established a rational in vitro screening cascade to analyse ADMET properties such as kinetic solubility/ logD7.4, metabolic stability/plasma stability in diverse species, permeability across cell layers, plasma protein binding and cytotoxicity. The applied technology includes state-of-the-art UHPLC-MS/MS (Triple Quad/Orbitrap), which further allows identification of metabolites.
Continuous screening of ADMET properties is guiding optimisation of compounds in-house as well as in collaboration with external groups. The results generated herein serve as a basis for the selection of compounds for in vivo studies, such as in our in-house zebrafish facility.