Saarbrücken, November 20, 2023 - Just like last year, the motto of World AMR Awareness Week 2023 is "Preventing antimicrobial resistance together". In the fight against resistant pathogens and their spread, successful collaboration plays a decisive role in several areas, including the development of new active substances. Especially the interface between academic research and clinical application is a critical point at which many projects fail. To address this gap, the Basel-based incubator INCATE specifically promotes projects with promising approaches to combating antimicrobial resistance. INCATE currently funds two projects at Helmholtz Institute for Pharmaceutical Research Saarland (HIPS): one on pathoblockers against Pseudomonas aeruginosa in Anna Hirsch's department and one on the natural product chlorotonil under the direction of Rolf Müller. In this interview, HIPS scientists Jörg Haupenthal, Jennifer Herrmann and Andreas Kany explain what the two projects are about.
New pathoblockers against Pseudomonas aeruginosa (Department DRUG DESIGN AND OPTIMISATION)
Jörg, your project is called Paerublock - how did the name come about and what does it stand for?
Jörg Haupenthal: This is an artificial word derived from the addressed pathogen "Pseudomonas aeruginosa" and the word "pathoblocker". P. aeruginosa is the pathogen we are addressing in our project, and pathoblocker is the name of the strategy behind our active substances, which are a newly developed alternative to conventional antibiotics.
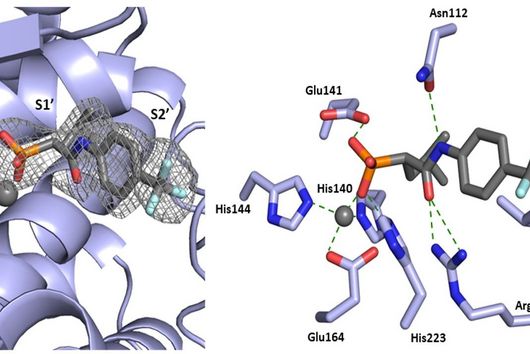
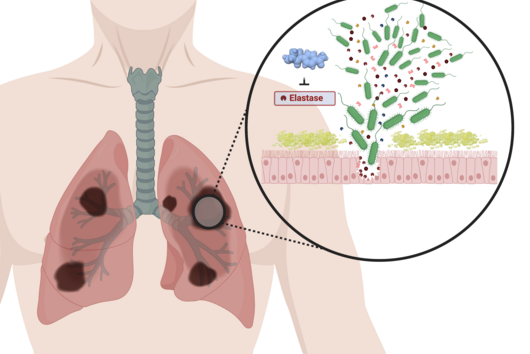
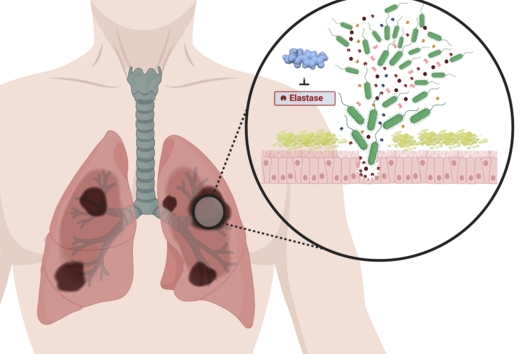
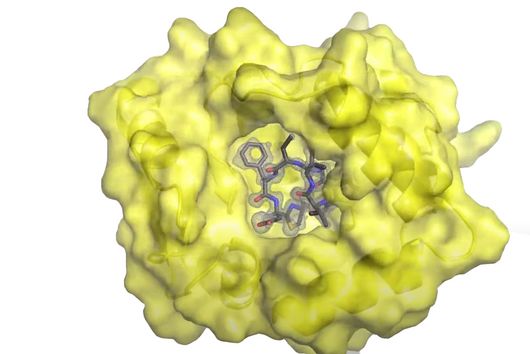
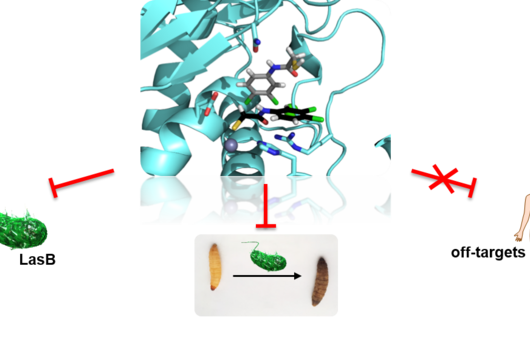
The active substances you have developed are not intended to act directly on the disease-causing bacteria themselves, but target a protein that is produced by these bacteria during an infection and released into the human body. How does this approach work?
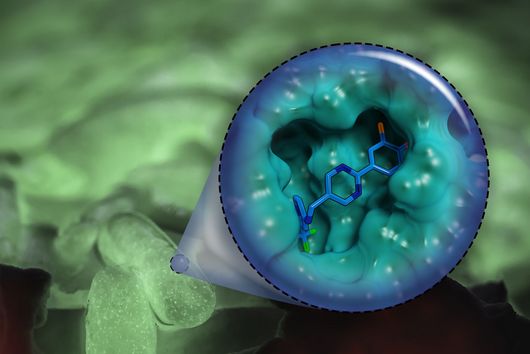
Jörg Haupenthal: While classical antibiotics target structures in or on the bacterium, we are interested in an enzyme that is produced and secreted by the pathogen – the so-called elastase. This enzyme is able to degrade a component of human tissue and thus helps the bacterium to penetrate the tissue, evade the immune response and obtain nutrients. Pharmaceutical inhibition of this enzyme does not kill the bacterium, but drastically reduces its pathogenic properties. This in turn makes it much easier for the immune system and classic antibiotics to deal with the germ. Hence the name pathoblocker - the aim is to attenuate or block the pathogenicity of the germ.
How far has Paerublock already progressed and how does the INCATE funding support you in further development?
Jörg Haupenthal: P. aeruginosa causes various infectious diseases and elastase therefore plays a role in various infected organs, such as the lungs and the eye. Our inhibitors are very potent, have so far not shown any toxicity and reach their target in sufficient quantities. Now, together with experts selected by INCATE, we need to put the finishing touches to the experimental tests. This should not only confirm the effectiveness in vivo, but also create therapeutic conditions that come as close as possible to those in humans. If successful, the first clinical trials will be prepared.
Optimisation of the natural product Chlorotonil (Department MICROBIAL NATURAL PRODUCTS)
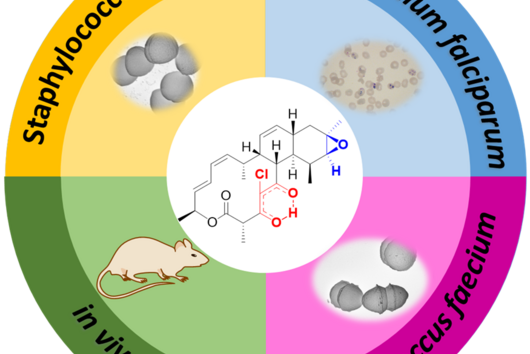
Jenni and Andreas, with the funding you have received from the INCATE incubator, you want to develop the natural product class of chlorotonils into an active ingredient against hospital germs. In September, HIPS also acquired additional funding from the BMBF's "GO-Bio initial" initiative - in this project, however, chlorotonils are to be used to treat malaria. How does this fit together?
Jennifer Herrmann: Chlorotonil antibiotics are extremely effective against important hospital pathogens such as vancomycin-resistant enterococci (VRE) and Methicillin-resistant Staphylococcus aureus (MRSA), as well as against the malaria pathogen Plasmodium falciparum. For this reason, we are working on a two-pronged project: on the one hand, we are working on optimising an antibiotic for the treatment of severe bacterial infections, supported by INCATE, and on the other hand, we are aiming to develop an antimalarial drug, which in turn is supported by GO-Bio initial. As these are fundamentally different indications, different aspects need to be addressed in each case, for example with regard to the infection models in animals. The requirements for the drug itself and its form of administration are also very different. For example, the treatment of malaria should primarily be carried out using tablets, whereas the treatment of severe bacterial infections in hospitals is usually carried out using intravenous administration. However, there are also synergies between the two branches of development, for example in the biotechnological production of the active ingredients, which we can use in both cases.
In addition to excellent antimicrobial activity, chlorotonils also have a novel mode of action. Can you explain why this is so important for new antibiotics?
Andreas Kany: Most new antibiotics that are currently in clinical trials have similar modes of action to antibiotics that are already on the market. This increases the risk that existing resistance mechanisms can be quickly adapted to the new antibiotics. However, our overarching goal is to counteract the increasing spread of antibiotic resistance by developing antibiotics with novel modes of action. In this context, we have so far only observed an extremely low trend towards the development of resistance for the chlorotonil antibiotics under laboratory conditions, which makes this substance class particularly promising.
How far has the development of this substance class progressed so far and what hurdles still need to be overcome before potential commercialization?
Andreas Kany: We are currently on the threshold of so-called lead structure optimization. This means that we have identified a very promising molecule from the chlorotonil class for which we have already been able to confirm its very good efficacy and tolerability in animal models. Further optimizations include, for example, the synthesis of even better derivatives and an in-depth understanding of their pharmacological and safety-relevant properties. Once we overcome these hurdles, we will select a candidate compound for which we will then aim for clinical development.